Medical device manufacturers face numerous challenges when bringing their innovative products to the market. From regulatory compliance to design and development, each stage requires meticulous attention to detail and expertise to be successful.
PolarSeal®, with its comprehensive solutions and industry experience, offers a streamlined approach to accelerate your medical device’s journey to market while ensuring quality and regulatory compliance across different territories.
In this article, we will explore how PolarSeal® can support you at every stage of the medical device development process and help expedite your path to market success .
Before delving into the details, let’s highlight the key takeaways:
Key takeaways:
- PolarSeal® offers a comprehensive solution for medical device manufacturers, streamlining the route to market and ensuring quality and regulatory compliance across different territories.
- PolarSeal® accelerates medical device development with rapid prototyping, streamlined manufacturing, in-house testing capabilities & tailored compliance strategies.
- With expertise in project discovery, research and development, manufacturing and packaging, product testing, and regulatory compliance, PolarSeal® supports manufacturers at every stage of the medical device development process.
- Services provided by PolarSeal® include project management, material and adhesive selection, prototyping, manufacturing in ISO 7 cleanrooms, product testing for reliability and safety, and assistance with regulatory requirements such as CE marking and FDA submissions.
By partnering with PolarSeal®, medical device manufacturers can expedite their journey to market, optimise their product development, and navigate the complex regulatory landscape with confidence.
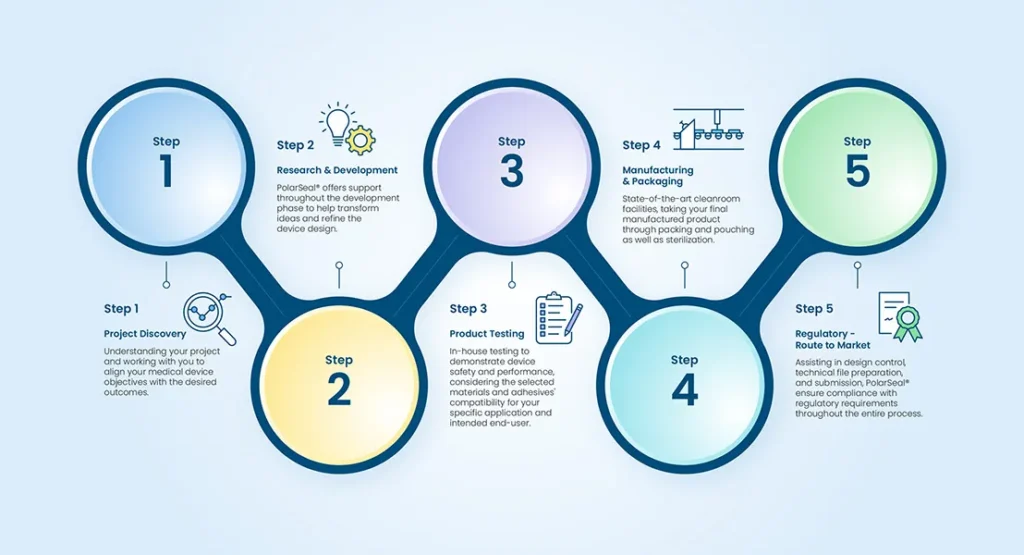
Project Discovery - Defining Your Medical Device Project Objectives
The success of any medical device project starts with a clear understanding of its objectives. PolarSeal® assists in project discovery by defining and aligning your medical device project objectives with the desired outcomes. This process involves:
Medical Device Ideation and Intended Use - Aligning Purpose with User Needs
To align the purpose of your device with user expectations, PolarSeal® emphasises the importance of gathering user feedback throughout the development process. By continuously adapting the device to evolving user needs, you can ensure that it addresses the specific requirements of your target audience effectively.
Materials Selection - Ensuring Optimal Performance and Safety
Selecting the right materials is crucial for the performance and safety of your medical device. PolarSeal® provides specialist support in material selection, particularly for skin-contacting devices and prototyping.
PolarSeal® offers expertise in compliant materials selection, avoiding substances such as CMR/EDC, rubber, and latex. When selecting materials, other considerations (depending on device requirements) include biocompatibility, sterilisation compatibility, and mechanical properties.
PolarSeal® also tests materials for suitability and regulatory compliance, ensuring manufacturability of material combinations to meet safety and performance criteria.
Medical Device Classification - Regulatory Compliance and Categorisation Guidelines
Regulatory compliance is a critical aspect of medical device development and keeping compliance considerations front of mind at every stage of device production helps ensure a simplified and efficient licensing process.
PolarSeal® guides you in understanding the classification criteria for medical devices, ensuring accurate categorisation. We provide expertise in complying with regulatory guidelines specific to each device class, helping navigate the medical device classification process effectively.

Medical Device Research and Development
Research and development are key stages in bringing a medical device from concept to reality. PolarSeal® offers support throughout this phase, focusing on two critical aspects:
1. Conceptual Design: Transforming Ideas into Functional Concepts
PolarSeal® facilitates the transformation of ideas into functional concepts by identifying user needs and defining design requirements. We generate innovative ideas and concept prototyping, evaluating the feasibility and selecting the most promising concept to move forward with.
2. Prototyping and Iterative Development: Refining the Device Design
Prototyping plays a crucial role in refining the device design and ensuring its functionality and user-friendliness. PolarSeal® assists in building early-stage prototypes to test functionality and ergonomics. User feedback is gathered, and the design is iterated upon to enhance performance and user experience.
Project Discovery - Defining Your Medical Device Project Objectives
Thorough testing is essential to ensure the reliability and safety of medical devices. PolarSeal® performs in-house testing to demonstrate device safety and performance, considering the selected materials and adhesives’ compatibility for your specific application and intended end-user.
Our focus on various aspects of testing include:
Regulatory Compliance Testing: Meeting Standards and Requirements
PolarSeal® places great importance on adhering to regulatory standards and guidelines. We conduct tests for compliance, ensuring that the device meets all necessary regulatory requirements. By obtaining the necessary certifications for market entry, PolarSeal® ensures your device’s compliance with the highest standards of safety and quality.
Performance Testing and Validation: Assessing Functionality and Reliability
PolarSeal® evaluates the device’s performance across different conditions to assess its functionality and reliability. We measure accuracy, precision, and response time while identifying potential issues that may impact functionality and reliability.
Other testing includes:
- Transit testing
- Performance tests (base layer adhesion/peel testing)
- Stability testing
- Packaging integrity testing
- Biological Safety Evaluation of Materials

Medical Device Manufacturing & Packaging
Once the medical device design and testing stages are complete, manufacturing and packaging come into play. PolarSeal® offers comprehensive support throughout this phase within our state-of-the-art ISO 7 class 10,000 cleanroom facilities, taking your final product through packing and pouching as well as sterilisation, providing a pre-market readiness solution.
PolarSeal®’s cleanrooms are meticulously maintained on both sites in accordance with ISO 14644:2015, ensuring the highest standards of cleanliness and control.
Scalable Manufacturing Solutions: Meeting Production Demands
PolarSeal® implements scalable manufacturing processes to meet production demands efficiently. We optimise workflow to ensure timely and cost-effective production. By leveraging technology and automation, PolarSeal® enhances productivity while maintaining the highest standards of quality.
Quality Control in Manufacturing: Ensuring Consistency and Reliability in Accordance with ISO 13485 and CFR cGMP
PolarSeal® prioritises quality control in medical device manufacturing, following ISO 13485 and CFR guidelines. We enforce stringent protocols, conducting thorough inspections and tests to maintain consistent quality. Through data analysis and prompt issue resolution, we ensure that each device meets the highest standards, instilling confidence and trust in both our clients and end-users.
Packaging and Labelling Design and Compliance: Protecting the Product and Enhancing User Experience
PolarSeal® understands the importance of packaging and labelling in protecting the device and enhancing the user experience. We design packaging solutions that effectively safeguard the medical device, complying with packaging and labelling regulations. By optimising packaging for user experience and safety, we create a positive interaction between users and your device.
Sterile Manufacturing Validation & Capabilities
Sterile manufacturing is crucial for certain medical devices. PolarSeal® operates ISO 7 cleanroom facilities and ensures sterile manufacturing validation according to ISO 13485 standards. We follow rigorous quality assurance processes, including process validation, PFMEA (Process Failure Mode and Effects Analysis), packaging validation, and sterilisation validation. Focusing on patient care and infection prevention, PolarSeal® offers a range of sterilization services including; E-Beam irradiation sterilization; EtO sterilization; and Gamma irradiation sterilization.

Regulatory Support Services for Navigating the Route to Market Compliance
PolarSeal® understands the complexity of the regulatory landscape and provides comprehensive support services to navigate the route to market compliance. We assist in design control, technical file preparation, and submission, ensuring compliance with regulatory requirements throughout the entire process.
Compliance Assessment: Evaluating Regulatory Requirements for Medical Devices
At PolarSeal®, we recognise the critical role of compliance assessment in successfully entering the market with medical devices. That’s why we provide dedicated assistance for compliance assessment. Our expert team conducts meticulous evaluations of the regulatory landscape to identify device-specific requirements based on classification, intended use, and target markets.
Remaining abreast of current regulatory developments is vital for preserving market access for medical devices. At PolarSeal®, we diligently monitor regulatory updates, industry trends, and emerging guidelines.
Our proactive approach enables us to adjust our strategies and offer timely guidance to our clients, guaranteeing their devices maintain compliance with the ever-evolving regulatory environment. By continuously tracking and interpreting regulatory changes, we provide customised strategies that align with the dynamic nature of the medical device industry.
Documentation Preparation: Crafting Comprehensive Technical Files and Submissions
We work closely with our clients to compile thorough and accurate technical files that comply with regulatory requirements, as well as:
- Create detailed technical documentation to support device safety and performance.
- Ensure accuracy and completeness for faster regulatory review and approval.
- Collaborate with regulatory experts to meet complex documentation requirements.
For the regulatory route to market, we can support compliant compilation of technical file submissions for example:
- (CE – MDR)
- MHRA UKCA (NI)
- FDA 510K
Post-Market Compliance: Ensuring Ongoing Regulatory Compliance and Surveillance
PolarSeal® provides comprehensive assistance with Post-Market Compliance, recognising the importance of maintaining regulatory adherence throughout the entire lifecycle of medical devices.
Our support includes establishing robust post-market surveillance systems for continuous medical device safety and performance monitoring. We assist in implementing effective mechanisms for adverse event reporting, complaint handling, and clinical follow-up, ensuring timely and appropriate actions.
We also understand the significance of keeping technical documentation up to date, regularly updating it along with risk management processes and labelling to meet evolving regulatory requirements.
With our ISO 13485 certification, ISO 7 cleanroom capability, and compliance with FDA 21 CFR Part 820, we assure clients that their devices meet the highest quality management standards and stringent regulatory requirements.
PolarSeal® offers a range of services for various market routes, including classification assistance, document preparation, and final submissions, as well as:
- UK Responsible Person Services (UKRP)
- CE Technical Documentation & Design Dossiers
- MDR technical file compilation (EU 2017/745)
- UKCA Marking
- CE MARKING
- Clinical Evaluation Report (CER) Writing
- Risk Management
- Post-Market Clinical Follow-up protocol (PMCF)
- Periodic Safety Updated Report (PSUR)
- US – FDA 510(k)s / De Novo Submissions
Contact PolarSeal® for your specific needs.

Case Study: 4 Weeks from Design to Market - How PolarSeal® Rapidly Brought a PPE Product to Market
Case Study: Personal Protective Equipment (PPE)
As an adhesive tape converter and component manufacturer, we provide solutions with our diverse range of capabilities. Our success stories highlight the transformative impact of our services on the medical device industry, showcasing our ability to navigate complex regulatory landscapes, ensure compliance, and drive market success. In this case study, we were able to help a client produce a high volume of PPE with rapid turnaround times.
Challenges Faced: | Solution Implemented: | Results Achieved: |
| The global pandemic triggered an urgent outcry for record speed design and production of PPE. The client came to PolarSeal® with the need to produce the correct requirements and volumes for a a single-face visor that was comfortable, adjustable, and produced under the correct medical device regulations. | We ensured the product was designed, process developed, and collated all materials that needed to be sourced for high-volume manufacture within 2 weeks. PolarSeal® manufactured the products, so they produce fully CE-compliant PPE and ensured it had been certified within 4 weeks. Highlighting how quickly our design and production service of PPE is. | Our team were able to engineer a product that allows for rigid fixation for our client, meeting the unique specifications provided. Working closely alongside the client, we were able to also find a solution to manufacture an economically viable product to produce. We were also able to aid with the CE marking and patent process. |

End-to-End Medical Device Manufacturing Support and Contract Manufacturing (CM)
Partnering with PolarSeal® empowers medical device manufacturers to expedite their path to market, achieve regulatory compliance, and overcome industry challenges.
With a comprehensive range of solutions and expertise in design, development, manufacturing, testing, and regulatory compliance, PolarSeal® offers end-to-end support throughout the entire device development process. With a track record of successful product launches, a commitment to quality, and streamlined processes, we are a trusted partner in efficiently and effectively bringing your medical device to market.
Choose PolarSeal® for accelerated success in your medical device journey. Start your journey with us today and learn more about what we can offer.
Read More from PolarSeal

7 complications when outsourcing to multiple vendors
The service offering of outsourcing your manufacturing process is a common practice within medical device manufacturing, by outsourcing the manufacture of your product you free

What makes our Leadership Team unique?
Family founded businesses hold a special place in the economic landscape, combining the strength of kinship with entrepreneurial spirit, PolarSeal possess a distinct character that

Route to market
Route to market for medical devices Quality and Regulatory getting you market ready PolarSeal® equips you with everything you need to take your medical device